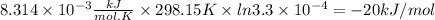Chemistry, 10.10.2019 00:30 idontknow1993
Acetylsalicylic acid (hc9h7o4) is a weak acid that dissociates in water in the following manner. hc9h7o4(aq) + h2o(l) equilibrium reaction arrow c9h7o4−(aq) + h3o+(aq) at a temperature of 298.15 k, the acid-dissociation constant (ka) for acetylsalicylic acid is 3.3 ✕ 10−4. (a) calculate the change in standard free energy (δg°) for this equilibrium reaction. kj/mol (b) what is the value of δg at equilibrium? δg > 0 δg = 0 δg < 0 (c) if the reaction were spontaneous in the forward direction, what type of value would you expect for δg?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:00
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
Acetylsalicylic acid (hc9h7o4) is a weak acid that dissociates in water in the following manner. hc9...
Questions






Health, 30.08.2019 12:10

Mathematics, 30.08.2019 12:10

Social Studies, 30.08.2019 12:20


History, 30.08.2019 12:20


Biology, 30.08.2019 12:20


Chemistry, 30.08.2019 12:20

Mathematics, 30.08.2019 12:20

Chemistry, 30.08.2019 12:20

Mathematics, 30.08.2019 12:20


Computers and Technology, 30.08.2019 12:20

Mathematics, 30.08.2019 12:20