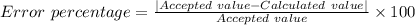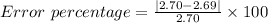Your calculated density of aluminum is d = 2.69 g/cm3. aluminum’s accepted density is 2.70 g/cm3. without writing the "%" sign, calculate the percent error up to two decimal places: your calculated density of aluminum is d = 2.69 g/cm3. aluminum’s accepted density is 2.70 g/cm3. without writing the "%" sign, calculate the percent error up to two decimal places:

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1
You know the right answer?
Your calculated density of aluminum is d = 2.69 g/cm3. aluminum’s accepted density is 2.70 g/cm3. wi...
Questions

Computers and Technology, 18.04.2020 02:03





Mathematics, 18.04.2020 02:03




Biology, 18.04.2020 02:03








Business, 18.04.2020 02:04

English, 18.04.2020 02:04