
Procedure for titrating an acid against a standard solution of naoh. the acid-base indicator, phenolphthalein, is colorless in acidic solution but takes on a pink color in basic solution. how would the volume of standard solution added change if that solution were ba(oh)2(aq) instead of naoh(aq)?

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1

Chemistry, 23.06.2019 10:20
An engineer wishes to design a container that will hold 12.0 mol of ethane at a pressure no greater than 5.00x10*2 kpa and a temperature of 52.0 degrees celsius. what is the minimum volume the container can have?
Answers: 1

Chemistry, 23.06.2019 15:00
What do we call the rows on the periodic table? a. periodb. familyc. groupd. metals
Answers: 1
You know the right answer?
Procedure for titrating an acid against a standard solution of naoh. the acid-base indicator, phenol...
Questions




English, 24.11.2019 00:31


Mathematics, 24.11.2019 00:31



Mathematics, 24.11.2019 00:31


Mathematics, 24.11.2019 00:31



Mathematics, 24.11.2019 00:31

Mathematics, 24.11.2019 00:31


English, 24.11.2019 00:31


Mathematics, 24.11.2019 00:31

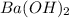 solution required will be half the volume required for NaOH solution.
solution required will be half the volume required for NaOH solution.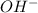 ion upon complete dissociation
ion upon complete dissociation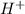 ion. But one molecule of
ion. But one molecule of 


