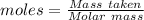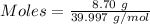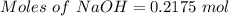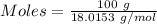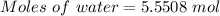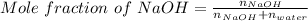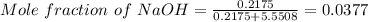Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
Asodium hydroxide solution is made by mixing 8.70 g naoh with 100 g of water. the resulting solution...
Questions



Biology, 24.03.2020 01:34

English, 24.03.2020 01:34

Mathematics, 24.03.2020 01:34


Computers and Technology, 24.03.2020 01:34

Mathematics, 24.03.2020 01:34

Chemistry, 24.03.2020 01:34

Mathematics, 24.03.2020 01:34

Chemistry, 24.03.2020 01:35






Computers and Technology, 24.03.2020 01:35


Computers and Technology, 24.03.2020 01:35

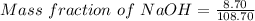 = 0.08004
= 0.08004