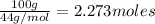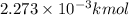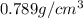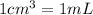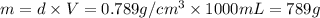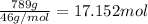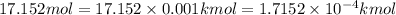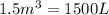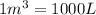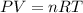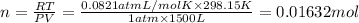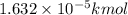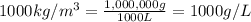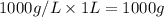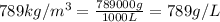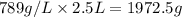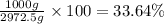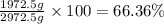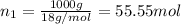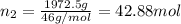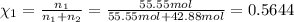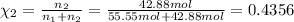How many moles, kmols in: 100 g of co2, 1 litre of ethyl alcohol of density 0.789 g/cm3 and a) 1.5m3 of o2 at 25°c and 1 atm. b) a mixture of water and ethyl alcohol is made up of 1 litre of water and 2.5 litre of alcohol. calculate the mass fraction and mol fraction for water and alcohol density of water 1000 kg/m density of alcohol 789 kg/m

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 05:50
Astudent made a graph plotting the progress of a reaction over time. the student forgot to label the y-axis of the graph. a graph is shown with two graph lines. one graph line starts at a higher position on the y axis and slopes downwards towards the right. the other graph line starts at a lower position on the y axis and slopes upwards towards the right. the two graph lines stop short of intersecting each other and continue as separate lines which gradually become straight and parallel to the x axis. a vertical line is shown at a point where the two graph lines finally became parallel to the x axis. this vertical line is labeled equilibrium. the title on the x axis is time and an arrow pointing towards the right is shown above time. the title on the y axis is left blank. what best explains the label that the student should use on the y-axis? amount, because as the amount of product decreases, the amount of reactant increases over time. reaction rate, because forward and backward reaction become equal at equilibrium. amount, because the amounts of reactants and products become constant after equilibrium is reached. reaction rate, as the rate of forward reaction increases and rate of backward reaction decreases over time.
Answers: 3

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1
You know the right answer?
How many moles, kmols in: 100 g of co2, 1 litre of ethyl alcohol of density 0.789 g/cm3 and a) 1.5m...
Questions

Arts, 12.01.2021 02:40

Health, 12.01.2021 02:40

Physics, 12.01.2021 02:40

Mathematics, 12.01.2021 02:40


Mathematics, 12.01.2021 02:40





Advanced Placement (AP), 12.01.2021 02:40


Mathematics, 12.01.2021 02:40

Social Studies, 12.01.2021 02:40


Social Studies, 12.01.2021 02:40

English, 12.01.2021 02:40


Mathematics, 12.01.2021 02:40

Mathematics, 12.01.2021 02:40