
In the lab you measure a clean dry crucible and cover to be 24.36 grams. you obtain a 2cm piece of pure magnesium metal. after sand papering the magnesium you place it in into your crucible and the mass of crucible, cover and magnesium masses out to be 24.66 grams. you observe that the magnesium is gray and shiny. you heat the crucible gently at first then vigorous for 10 minutes until the magnesium ignites. after the crucible cools you add a few drops of distilled water and heat again. the mass of the magnesium compound, crucible, and cover masses out to be 24.85 grams.
what is the empirical formula of the compound? show all work and units.
1) mass of the mg
2) mass of the mgo
3) mass of 0
4) moles of mg & moles of o
5) show mole to mole mgxoy
6) empricial formula of compound

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the formula for the molecular compound nitrogen monoxide
Answers: 1

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1
You know the right answer?
In the lab you measure a clean dry crucible and cover to be 24.36 grams. you obtain a 2cm piece of p...
Questions







Computers and Technology, 12.08.2021 22:20



Mathematics, 12.08.2021 22:20

Mathematics, 12.08.2021 22:20


Computers and Technology, 12.08.2021 22:20



Mathematics, 12.08.2021 22:20

Mathematics, 12.08.2021 22:20



French, 12.08.2021 22:20

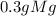 *
*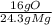 = 0.2g O
= 0.2g O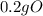 *
*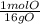 = 0.01mol O
= 0.01mol O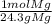 = 0.01mol Mg
= 0.01mol Mg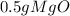 *
*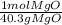 = 0.01mol MgO
= 0.01mol MgO

