
Chemistry, 10.10.2019 03:30 Imamdiallo18
Consider the reaction 2 x2y + z2 ⇌ 2 x2yz which has a rate law of rate= k[x2y][z2] select a possible mechanism for the reaction. group of answer choices
step 1: z2 --> z + z (slow) step 2: x2y + z → x2yz (fast) step 3: x2y + z → x2yz (fast) step 1: x2y + z2→ x2yz2 (slow) step 2: x2yz2 → x2yz + z (fast) step 1: 2 x2y + z2 → 2x2yz (slow) step 1: x2y + z2 → x2yz + z (slow) step 2: x2y + z → x2yz (fast) step 1: 2 x2y ⇌ x4y2 (fast) step 2: x4y2 + z2 → 2 x2yz (slow)

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1

Chemistry, 23.06.2019 07:30
In the diagram here that represents the reaction, which reactant, a or b, is the limiting reagent?
Answers: 1

Chemistry, 23.06.2019 09:30
If the solubility of a gas in water is 1.22g/2.75 atm, what is it’s solubility (in g/l) at 1.0 atm
Answers: 1

Chemistry, 23.06.2019 10:00
Two moles of potassium chloride and three moles of oxygen are produced from the decomposition of two moles of potassium chlorate, kcos3(s). write the balanced equation. how many moles of oxygen are produced from 12 moles of potassium chlorate
Answers: 1
You know the right answer?
Consider the reaction 2 x2y + z2 ⇌ 2 x2yz which has a rate law of rate= k[x2y][z2] select a possible...
Questions


Social Studies, 08.12.2020 01:20

Mathematics, 08.12.2020 01:20




History, 08.12.2020 01:20

Arts, 08.12.2020 01:20

Mathematics, 08.12.2020 01:20




English, 08.12.2020 01:20


Mathematics, 08.12.2020 01:20


Mathematics, 08.12.2020 01:20

Mathematics, 08.12.2020 01:20

World Languages, 08.12.2020 01:20

Mathematics, 08.12.2020 01:20

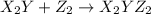 (slow)
(slow)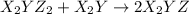 (fast)
(fast)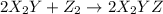
![Rate=k[X_2Y][Z_2]](/tpl/images/0305/6835/e6034.png)
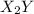 and
and 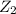 .
.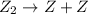 (slow)
(slow)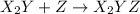 (fast)
(fast)![Rate=K[Z_2]](/tpl/images/0305/6835/c66c5.png)
![Rate=k[X_2Y]^2[Z_2]](/tpl/images/0305/6835/8a2d6.png)
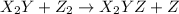 (fast)
(fast)![Rate=K'[X_2Y][Z]](/tpl/images/0305/6835/3c7d4.png) .............(1)
.............(1)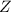 , we get:
, we get:![\frac{d[Z]}{dt}=K"[X_2Y][Z_2]](/tpl/images/0305/6835/87d2e.png) .........(2)
.........(2)![Rate=K'K"[X_2Y]^2[Z_2]](/tpl/images/0305/6835/e4aa1.png)
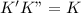
![Rate=K[X_2Y]^2[Z_2]](/tpl/images/0305/6835/5d2a6.png)
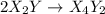 (fast)
(fast)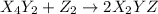 (slow)
(slow)![Rate=K'[X_4Y_2][Z_2]](/tpl/images/0305/6835/57b59.png) .............(1)
.............(1)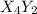 , we get:
, we get:![\frac{d[X_4Y_2]}{dt}=K"[X_2Y]^2](/tpl/images/0305/6835/bce7e.png) .........(2)
.........(2)

