
Chemistry, 10.10.2019 04:10 princess42044
Achemist obtains 500.0 ml of a solution containing an unknown concentration of calcium iodide, cai 2. he pipets 20 ml of this solution into a 100 ml volumetric flask and dilutes to the mark. he then pipets 10 ml of this diluted solution into a 100 ml volumetric flask and dilutes to the mark. he analyzes some of the solution from the final volumetric flask and finds that the iodide ion concentration is 0.574 m. (note: in solution, calcium iodide breaks apart into one ca 2+ ion for every two i - ions, so a solution that is 1.0 m in cai 2 is 2.0 m in i determine the molar concentration of calcium iodide in the original solution.

Answers: 3
Another question on Chemistry




Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3
You know the right answer?
Achemist obtains 500.0 ml of a solution containing an unknown concentration of calcium iodide, cai 2...
Questions

Mathematics, 03.12.2019 04:31






Advanced Placement (AP), 03.12.2019 04:31





Chemistry, 03.12.2019 04:31

History, 03.12.2019 04:31

Mathematics, 03.12.2019 04:31


Mathematics, 03.12.2019 04:31

Biology, 03.12.2019 04:31

Biology, 03.12.2019 04:31

History, 03.12.2019 04:31

Mathematics, 03.12.2019 04:31

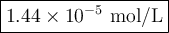
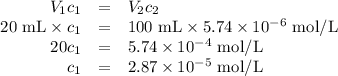
![[\text{Ca}^{2+}] =\dfrac{2.87 \times 10^{-5} \text{ mol I}^{-}}{\text{1 L}} \times \dfrac{\text{1 mol Ca}^{2+} }{\text{2 mol I}^{-}} = \mathbf{1.44 \times 10^{-5}} \textbf{ mol/L}\\\\\text{[Ca$^{2+}$] in the original solution was $\large \boxed{\mathbf{1.44 \times 10^{-5}} \textbf{ mol/L}}$}](/tpl/images/0305/8108/dfb7a.png)


