
Chemistry, 10.10.2019 05:00 akaeiraspruell
Use the following equilibrium reaction and constant for the deprotonation of bicarbonate (hco3-) to carbonate (co32-) to determine: hco3 = co2 + h+ k = 10-10.33 (a) whether hco3 or co32- would dominate at ph 9.1 and (b) what the concentration of [co32-] would be at this ph if [hco3 ] = 10-6 m

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2


Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

You know the right answer?
Use the following equilibrium reaction and constant for the deprotonation of bicarbonate (hco3-) to...
Questions

Mathematics, 16.07.2019 23:00


Chemistry, 16.07.2019 23:00



Mathematics, 16.07.2019 23:00

Biology, 16.07.2019 23:00


Mathematics, 16.07.2019 23:00

History, 16.07.2019 23:00

Mathematics, 16.07.2019 23:00

Mathematics, 16.07.2019 23:00

History, 16.07.2019 23:00

English, 16.07.2019 23:00





Biology, 16.07.2019 23:00

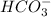 will dominate at pH = 9.1
will dominate at pH = 9.1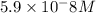
![pH=-\log[H^+]](/tpl/images/0305/9437/cf945.png) ......(1)
......(1)![9.1=-\log[H^+]](/tpl/images/0305/9437/e5ddb.png)
![[H^+]=10^{-9.1}](/tpl/images/0305/9437/b7f9d.png)
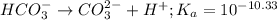
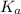 for above reaction follows:
for above reaction follows:![K_a=\frac{[CO_3^{2-}]\times [H^+]}{[HCO_3^-]}](/tpl/images/0305/9437/fea1b.png)
![10^{-10.33}=\frac{[CO_3^{2-}]\times 10^{-9.1}}{[HCO_3^-]}\\\\\frac{[HCO_3^-]}{[CO_3^{2-}]}=\frac{10^{-9.1}}{10^{-10.33}}\\\\\frac{[HCO_3^-]}{[CO_3^{2-}]}=16.98](/tpl/images/0305/9437/786f2.png)
![[HCO_3^-]=16.98\times [CO_3^{2-}]](/tpl/images/0305/9437/e6ce5.png)
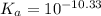
![[HCO_3^-]=10^{-6}M](/tpl/images/0305/9437/1335d.png)
![10^{-10.33}=\frac{[CO_3^{2-}]\times 10^{-9.1}}{10^{-6}}](/tpl/images/0305/9437/27401.png)
![[CO_3^{2-}]=\frac{10^{-6}\times 10^{-10.33}}{10^{-9.1}}=5.9\times 10^-8}M](/tpl/images/0305/9437/65347.png)


