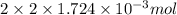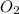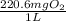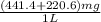Chemistry, 10.10.2019 05:00 geunagillis1
Determine the carbonaceous and nitrogenous oxygen demand in mg/l for a 1 l solution containing 300 mg of a wastewater represented by the formula cn2h602 (n is converted to nh3 in the first step) afterwards calculate the cod of the solution

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1


Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1
You know the right answer?
Determine the carbonaceous and nitrogenous oxygen demand in mg/l for a 1 l solution containing 300 m...
Questions






Arts, 06.06.2020 22:01


German, 06.06.2020 22:01

Mathematics, 06.06.2020 22:01


Mathematics, 06.06.2020 22:01









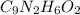 .
.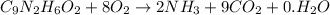
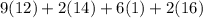
 mg/mol (as 1 g = 1000 mg)
mg/mol (as 1 g = 1000 mg)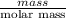
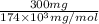
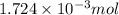
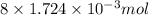
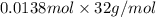
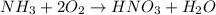
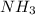 to convert into
to convert into 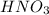 , oxygen required is 2 mol.
, oxygen required is 2 mol.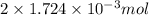 of
of