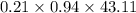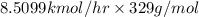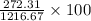Chemistry, 10.10.2019 05:00 cgattis6935
Amixture of a and b is capable of being ignited only if the mole percent of a is 6 %. a mixture containing 9.0 mole% a in b flowing at a rate of 800 kg/h is to be diluted with pure b to reduce a concentration to the lower flammability limit. calculate the required flow rate of b in mol/h and the percent by mass of oz in the product gas. (note: b may be taken to consist of 21 mole% o2 and 79% nz and to have an average molecular weight of 29.0.) ma = 16.04 g/mol.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1


Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

You know the right answer?
Amixture of a and b is capable of being ignited only if the mole percent of a is 6 %. a mixture cont...
Questions


Arts, 01.08.2019 17:30

Mathematics, 01.08.2019 17:30

Mathematics, 01.08.2019 17:30



Mathematics, 01.08.2019 17:30



Spanish, 01.08.2019 17:30





Mathematics, 01.08.2019 17:30


Mathematics, 01.08.2019 17:30



Business, 01.08.2019 17:30

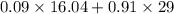
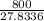
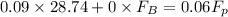
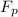 = 43.11 kmol/hr
= 43.11 kmol/hr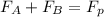
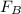 = 43.11 - 28.74
= 43.11 - 28.74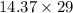
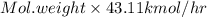
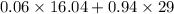
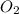 into the product stream is as follows.
into the product stream is as follows.