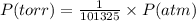Chemistry, 10.10.2019 05:20 hillarytrinh
495 cm3 of oxygen gas at 25 oc and 114,700 pa, and 877 cm3 of nitrogen gas 25.0 °c and 114,700 pa, are injected into an evacuated 536 cm3 flask.
a. find the number of moles of oxygen present prior to mixing the gases, assuming the temperature remains constant, and that oxygen is an ideal gas. (5pts)
b. find the number of moles of nitrogen present prior to mixing the gases, assuming the temperature remains constant and that nitrogen is an ideal gas. (5pts)
c. find the total pressure of oxygen and nitrogen in the flask (after the two gases are mixed together.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

Chemistry, 23.06.2019 05:30
How many moles are in 1.26*10^24 particles in significant figures
Answers: 2

Chemistry, 23.06.2019 05:50
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1
You know the right answer?
495 cm3 of oxygen gas at 25 oc and 114,700 pa, and 877 cm3 of nitrogen gas 25.0 °c and 114,700 pa, a...
Questions


Mathematics, 22.03.2021 21:00

English, 22.03.2021 21:00

Mathematics, 22.03.2021 21:00

Mathematics, 22.03.2021 21:00


Biology, 22.03.2021 21:00


English, 22.03.2021 21:00


Social Studies, 22.03.2021 21:00

Mathematics, 22.03.2021 21:00




Health, 22.03.2021 21:00



Mathematics, 22.03.2021 21:00