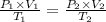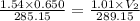Abubble of helium gas has a volume of 0.650 cm3 near the bottom of a large aquarium where the pressure is 1.54 atm and the temperature is 12°c. determine the bubble’s volume upon rising near the top where the pressure is 1.01 atm and 16°c. assume that the number of moles of helium remains constant and that the helium is an ideal gas. (13 pts)
important equations and constants 1 atm = 760 torr = 760 mmhg = 101,325 pa
1ml = 1cm3
pv = nrt
p1v1 = p2v2
v1/t1 = v2/t2
v1/n1 = v2/n2
ptotal = p1 + p2 + p3 + ….
(p1v1)/(n1t1) = (p2v2)/(n2t2)
r = 0.08206 l atm/mol k

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 08:00
Straightforward questions answered in the powerpoint slidesreaction: heating the starting materials under refluxwhat does it mean to heat under reflux? why do we choose water as the reflux solvent? what are boiling chips used for? why do we put a condenser on top of the reaction? why do we add heat and let the reaction stir for 30 minutes? why do we add sulfuric acid to the reaction after it cools as opposed to when it’s still hot? separation: filtration of precipitatewhy don’t we do an aqueous and organic extraction in the separatory funnel? why do you rinse the salicylic acid on the filter with ice cold water? purification: recrystallization of salicylic acid (no hot filtration needed)what is the difference in the amount of room temperature water vs. boiling water needed to dissolve the salicylic acid (assume a 1.2 gram yield of salicylic acid)? remember, in the lab if you need x ml of boiling water to dissolve a solid, then you should add a little more (definitely no more than 1.5 times the theoretical amount) to ensure it doesn’t recrystallize prematurely.analysis: melting point of salicylic acidwhat can you conclude if the melting point of the salicylic acid you just synthesized is 152-155oc and the 1: 1 mix of your product and “synthetic” salicylic acid is 151-154oc?
Answers: 1
You know the right answer?
Abubble of helium gas has a volume of 0.650 cm3 near the bottom of a large aquarium where the pressu...
Questions

World Languages, 20.01.2021 23:00

History, 20.01.2021 23:00

History, 20.01.2021 23:00

History, 20.01.2021 23:00


Mathematics, 20.01.2021 23:00


Mathematics, 20.01.2021 23:00

History, 20.01.2021 23:00

Mathematics, 20.01.2021 23:00


History, 20.01.2021 23:00


Mathematics, 20.01.2021 23:00

History, 20.01.2021 23:00

Mathematics, 20.01.2021 23:00

Mathematics, 20.01.2021 23:00

Mathematics, 20.01.2021 23:00

History, 20.01.2021 23:00

Mathematics, 20.01.2021 23:00