

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 21:30
Electromagnets coils of wire paper clips picked up 10 3 15 6 20 9 25 12 ms. owens' class was studying magnets. ms. owens showed her students how to make an electromagnet using a nail, a d-cell battery, and plastic coated wire. the students wrapped the wire around the nail and then attached the ends to the battery. when they were finished, they tested their magnets by investigating how many paperclips their magnets could pick up. they also tested whether they could increase the strength of their electromagnets by using more coils of wire. they recorded the class average of their results in the data table seen here. ms. owens asked her students to graph their data in a line graph. how should the students label the x-axis on their line graph? a) size of battery b) number of paper clips c) number of coils of wire d) strength of electromagnet
Answers: 2


Chemistry, 23.06.2019 04:10
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2

Chemistry, 23.06.2019 06:00
In an exothermic reaction at equilibrium, what is the effect of lowering the temperature? a. the reaction makes more products. b. the reaction makes more reactants. c. the reaction is unchanged.
Answers: 1
You know the right answer?
What is the percent by mass of oxygen in a gaseous mixture whose molar composition is 0.500 % co2 an...
Questions


English, 17.11.2020 22:20

Mathematics, 17.11.2020 22:20

Chemistry, 17.11.2020 22:20






Social Studies, 17.11.2020 22:20

Physics, 17.11.2020 22:20

Advanced Placement (AP), 17.11.2020 22:20

Mathematics, 17.11.2020 22:20


English, 17.11.2020 22:20

Mathematics, 17.11.2020 22:20

Mathematics, 17.11.2020 22:20

Biology, 17.11.2020 22:20

Mathematics, 17.11.2020 22:20

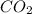 = 0.500 %
= 0.500 %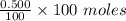 = 0.5 moles
= 0.5 moles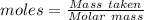
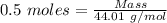
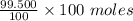 = 99.500 moles
= 99.500 moles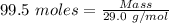
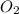 = 21 % of air
= 21 % of air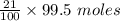 = 20.895 moles
= 20.895 moles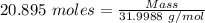
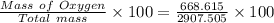 = 23.0 %
= 23.0 %

