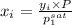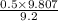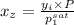Chemistry, 10.10.2019 05:30 aleahnew36
Aclosed system contains an equimolar mixture of n-pentane and isopentane. suppose the system is initially all liquid at 120°c and a high pressure, and the pressure is gradually reduced at a constant temperature. estimate the pressures at which the first bubble of vapor forms and at which the last drop of liquid evaporates. also calculate the liquid and vapor compositions (mole fractions) at those two conditions.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Rutherford's experiment indicated that matter was not as uniform as it appears what part of his experimental results implied this idea
Answers: 1

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

You know the right answer?
Aclosed system contains an equimolar mixture of n-pentane and isopentane. suppose the system is init...
Questions

Health, 16.04.2021 16:30

Mathematics, 16.04.2021 16:30

Mathematics, 16.04.2021 16:30

Physics, 16.04.2021 16:30


Biology, 16.04.2021 16:30




Mathematics, 16.04.2021 16:30

Mathematics, 16.04.2021 16:30

Computers and Technology, 16.04.2021 16:30


Mathematics, 16.04.2021 16:30



Biology, 16.04.2021 16:30


Mathematics, 16.04.2021 16:30

Mathematics, 16.04.2021 16:30

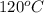 = (120 + 273.15)K = 393.15 K,
= (120 + 273.15)K = 393.15 K, 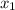 = 0.5 and
= 0.5 and 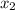 = 0.5
= 0.5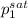 (393.15 K) = 9.2 bar
(393.15 K) = 9.2 bar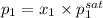
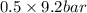
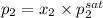
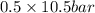
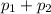
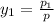
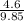
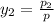
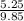
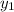 = 0.5,
= 0.5, 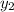 = 0.5
= 0.5 
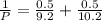
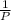 = 0.101966
= 0.101966 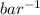
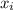 and its formula is as follows.
and its formula is as follows.