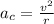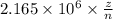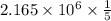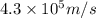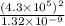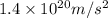Consider the hydrogen atom as described by the bohr model. the nucleus of the hydrogen atom is a single proton. the electron rotates in a circular orbit about this nucleus. in the n = 5, orbit the electron is 1.32 10-9 m from the nucleus and it rotates with an angular speed of 3.30 1014 rad/s. determine the electron's centripetal acceleration in m/s2.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2
You know the right answer?
Consider the hydrogen atom as described by the bohr model. the nucleus of the hydrogen atom is a sin...
Questions

Biology, 30.01.2020 11:02

Chemistry, 30.01.2020 11:02



Mathematics, 30.01.2020 11:02


Social Studies, 30.01.2020 11:02


History, 30.01.2020 11:02

Mathematics, 30.01.2020 11:02

Mathematics, 30.01.2020 11:02







Chemistry, 30.01.2020 11:02

Mathematics, 30.01.2020 11:02

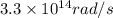 .
.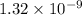 m
m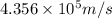
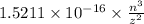 sec
sec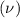 =
= 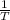
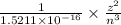
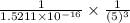
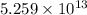 Hz
Hz