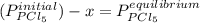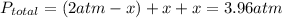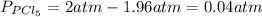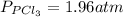Chemistry, 10.10.2019 18:10 lailabirdiemae
Aflask is filled with pcl5 to a pressure of 2.00 atm at 300°c and allowed to come to equilibrium according to the reaction: pcl5(g) ⇄ pcl3(g) + cl2(g) analysis shows the total pressure in the flask at equilibrium is 3.96 atm. calculate the equilibrium constant kp for the reaction.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1


You know the right answer?
Aflask is filled with pcl5 to a pressure of 2.00 atm at 300°c and allowed to come to equilibrium acc...
Questions


History, 21.03.2020 03:09







Biology, 21.03.2020 03:09

Chemistry, 21.03.2020 03:09

Mathematics, 21.03.2020 03:09

Mathematics, 21.03.2020 03:09

Biology, 21.03.2020 03:09


Mathematics, 21.03.2020 03:09




Biology, 21.03.2020 03:09

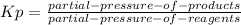
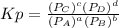
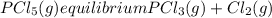
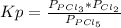
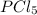 so the total pressure for the system is also the partial pressure for
so the total pressure for the system is also the partial pressure for 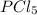 ,
,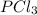 and
and 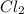 so now we need to estimate the partial pressure for each specie.
so now we need to estimate the partial pressure for each specie. 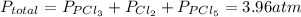
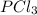 and
and 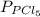 in the equilibrium will be
in the equilibrium will be