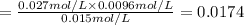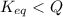At 1173 k, keq = 0.0108 for the following reaction: caco3(s) ⇄ cao(s) + co2(g) the reaction takes place in a 10.0 l vessel at 1173 k. if a mixture of 15.0 g caco3, 15.0 g cao, and 4.25 g co2 is allowed to approach equilibrium, what will happen to the amount of caco3? group of answer choices
- it will remain the same
- it will increase
- not enough information is provided to answer this question
- it will decrease

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 23.06.2019 01:30
List and describe the neurological effects of the vocs and other air pollutants,as described by dr.theo colborn
Answers: 2

Chemistry, 23.06.2019 03:30
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3

Chemistry, 23.06.2019 04:10
What does the field of thermodynamics relate to a-changes in nuclear reactions b- changes in energy in systems c changes in molecular structure d changes in atomic properties
Answers: 1
You know the right answer?
At 1173 k, keq = 0.0108 for the following reaction: caco3(s) ⇄ cao(s) + co2(g) the reaction takes p...
Questions





Social Studies, 26.11.2019 20:31



English, 26.11.2019 20:31


Social Studies, 26.11.2019 20:31

Mathematics, 26.11.2019 20:31





Biology, 26.11.2019 20:31

History, 26.11.2019 20:31

Computers and Technology, 26.11.2019 20:31


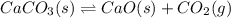
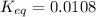
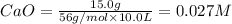
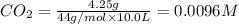
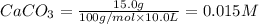
![Q=\frac{[CaO][CO_2]}{[CaCO_3]}](/tpl/images/0308/1118/76f7b.png)