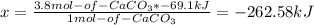Chemistry, 10.10.2019 22:10 BaileyElizabethRay
Calcium hydroxide, which reacts with carbon dioxide to form calcium carbonate, was used by the ancient romans as mortar in stone structures. the reaction for this process is ca(oh)2(s) + co2(g) → caco3(s) + h2o(g) δh = –69.1 kj what is the enthalpy change if 3.8 mol of calcium carbonate is formed?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Maria wants to determine which type of disinfectant kills the most bacteria. which of the following is the best way for maria to determine this? a. ask ten different companies that make disinfectants which type is best. b. put the same amount and species of bacteria on ten identical plates, and add ten different kinds of disinfectant to each plate. c. interview ten different people to determine which type of disinfectant they prefer. d. put the same amount and species of bacteria on ten identical plates, and add a different disinfectant to each plate.
Answers: 1

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
You know the right answer?
Calcium hydroxide, which reacts with carbon dioxide to form calcium carbonate, was used by the ancie...
Questions


Biology, 06.10.2019 09:02










Mathematics, 06.10.2019 09:10



Mathematics, 06.10.2019 09:10

Biology, 06.10.2019 09:10

History, 06.10.2019 09:10




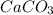 are formed.
are formed.