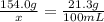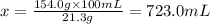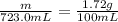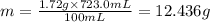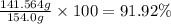The solubility of solid w in water is: 1.72 g/100 ml at 0°c, 21.3/100 ml at 100°c. a) how many ml of boiling water are required to dissolve 154.0 g of w? (report to the nearest ml) if solution were cooled to 0°c, how many grams of w would crystallize out? (report to one decimal place) b) what is the percent recovery? (report to one decimal place) (show calculations)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2


Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2
You know the right answer?
The solubility of solid w in water is: 1.72 g/100 ml at 0°c, 21.3/100 ml at 100°c. a) how many ml o...
Questions



Mathematics, 30.01.2022 05:20

Mathematics, 30.01.2022 05:20

Mathematics, 30.01.2022 05:20


History, 30.01.2022 05:20

Mathematics, 30.01.2022 05:20

Geography, 30.01.2022 05:20




Mathematics, 30.01.2022 05:30

Mathematics, 30.01.2022 05:30


Advanced Placement (AP), 30.01.2022 05:30


Chemistry, 30.01.2022 05:30