
Chemistry, 11.10.2019 00:00 jackfrost5
Astandard solution of fescn2+ is prepared by combining 9.0 ml of 0.20 m fe(no3)3 with 1.0 ml of 0.0020 m kscn . the standard solution had an absorbance of 0.480 . fe3+(aq)+scn−(aq)↽−−⇀fescn2+(aq) a trial solution was made in a similar manner, but with a more dilute fe(no3)3 reagent. the initial scn− concentration, immediately after mixing, was 0.00050 m . this trial solution had absorbance of 0.220 . what is the equilibrium concentration of scn− in the trial solution?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

You know the right answer?
Astandard solution of fescn2+ is prepared by combining 9.0 ml of 0.20 m fe(no3)3 with 1.0 ml of 0.00...
Questions

Mathematics, 21.05.2021 01:00


Arts, 21.05.2021 01:00


Mathematics, 21.05.2021 01:00

Mathematics, 21.05.2021 01:00

English, 21.05.2021 01:00











Mathematics, 21.05.2021 01:00


Mathematics, 21.05.2021 01:00

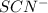 in the trial solution is
in the trial solution is 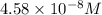
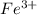 and
and 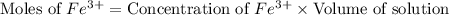
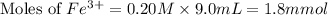
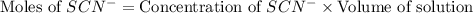
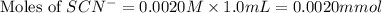
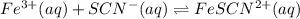
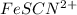
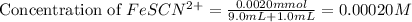

 = molar absorptivity coefficient
= molar absorptivity coefficient
![[SCN^-]_{eqm}=[SCN^-]_{initial}-[FeSCN^{2+}]](/tpl/images/0308/4929/92d10.png)
![[SCN^-]_{initial}](/tpl/images/0308/4929/bc58d.png) = 0.00050 M
= 0.00050 M![[FeSCN^{2+}]](/tpl/images/0308/4929/797d4.png) .
.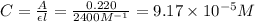
![[SCN^-]_{eqm}=(0.00050M)-(9.17\times 10^{-5}M)](/tpl/images/0308/4929/c2881.png)
![[SCN^-]_{eqm}=4.58\times 10^{-8}M](/tpl/images/0308/4929/1e6f2.png)


