Chemistry, 10.10.2019 23:30 makennahudson94
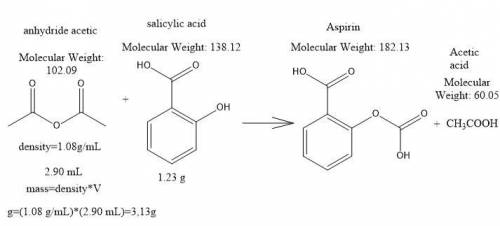
Aspirin can be made in the laboratory by reacting acetic anhydride (c4h6o3) with salicylic acid (c7h6o3) to form aspirin (c9h8o4) and acetic acid (c2h4o2). the balanced equation is
c4h6o3+c7h6o3→c9h8o4+c2h4o2
in a laboratory synthesis, a student begins with 2.90 ml of acetic anhydride (density=1.08gml−1) and 1.23 g of salicylic acid. once the reaction is complete, the student collects 1.24 g of aspirin.
1. determine the theoretical yield of aspirin for the reaction. express your answer using three significant figures.
2. determine the percent yield of aspirin for the reaction. express your answer using three significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
You know the right answer?
Aspirin can be made in the laboratory by reacting acetic anhydride (c4h6o3) with salicylic acid (c7h...
Questions

Mathematics, 30.08.2020 19:01

Mathematics, 30.08.2020 19:01




History, 30.08.2020 19:01

English, 30.08.2020 19:01

Biology, 30.08.2020 19:01





Mathematics, 30.08.2020 19:01

Mathematics, 30.08.2020 19:01

Biology, 30.08.2020 19:01


Mathematics, 30.08.2020 19:01

Mathematics, 30.08.2020 19:01

Mathematics, 30.08.2020 19:01

English, 30.08.2020 19:01