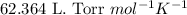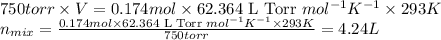Some commercial drain cleaners contain two components: sodium hydroxide and aluminum in the form of a powder. when the mixture is poured down a clogged drain, the following redox reactions occurs: 2naoh(aq) + 2 al(s) + 6h2o(l) → 2naal(oh)4(aq) + 3 h2(g) the heat generated in this reaction melt away grese and the hydrogen gas released stirs up the solids clagging the drain. calculate the volume hydrogen gas formed at 20. ºc and 750. torr if 3.12 g of al is treated with excess naoh.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
If a bottle of olive oil contains 1.4 kg of olive oil, what is the volume, in milliliters ( ml ), of the olive oil?
Answers: 1

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1
You know the right answer?
Some commercial drain cleaners contain two components: sodium hydroxide and aluminum in the form of...
Questions

History, 23.03.2021 05:10








Biology, 23.03.2021 05:10

Health, 23.03.2021 05:10

Mathematics, 23.03.2021 05:10




Mathematics, 23.03.2021 05:10

Social Studies, 23.03.2021 05:10

Mathematics, 23.03.2021 05:10



Biology, 23.03.2021 05:10

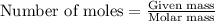
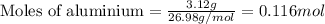
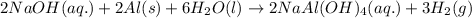
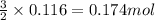 of hydrogen gas
of hydrogen gas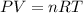
![20^oC=[20+273]K=293K](/tpl/images/0308/3868/3b5d4.png)