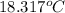Aquantity of 85.0 ml of 0.900 m hcl is mixed with 85.0 ml of 0.900 m koh in a constantpressure calorimeter that has a heat capacity of 325 j/°c. if the initial temperatures of both solutions are the same at 18.24°c, what is the final temperature of the mixed solution? the heat of neutralization is −56.2 kj/mol. assume the density and specific heat of the solutions are the same as those for water.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2
You know the right answer?
Aquantity of 85.0 ml of 0.900 m hcl is mixed with 85.0 ml of 0.900 m koh in a constantpressure calor...
Questions

Mathematics, 18.02.2021 19:30

English, 18.02.2021 19:30



Mathematics, 18.02.2021 19:30



Mathematics, 18.02.2021 19:30

Chemistry, 18.02.2021 19:30

Mathematics, 18.02.2021 19:30

Computers and Technology, 18.02.2021 19:30


Mathematics, 18.02.2021 19:30

Chemistry, 18.02.2021 19:30


Mathematics, 18.02.2021 19:30

Computers and Technology, 18.02.2021 19:30



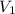 = 85.0 ml,
= 85.0 ml, 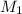 = 0.9 M
= 0.9 M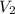 = 85.0 ml,
= 85.0 ml, 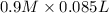 (as 1 L = 1000 ml so, 85 ml = 0.085 L)
(as 1 L = 1000 ml so, 85 ml = 0.085 L)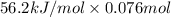
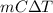
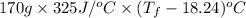
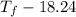
 =
=