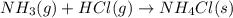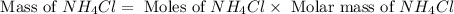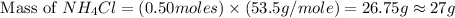For each of the following balanced chemical equations, calculate how many moles and how many grams of each product would be produced by the complete conversion of 0.50 mole of the reactant indicated in boldface. state clearly the mole ratio used for each conversion. a. nh3(g) 1 hcl(g) s nh4cl(s)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3


Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1
You know the right answer?
For each of the following balanced chemical equations, calculate how many moles and how many grams o...
Questions

Social Studies, 08.12.2021 21:10





Social Studies, 08.12.2021 21:10



Business, 08.12.2021 21:20






Mathematics, 08.12.2021 21:20


English, 08.12.2021 21:20

Chemistry, 08.12.2021 21:20


Spanish, 08.12.2021 21:20

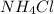 and mass of
and mass of 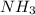 . So,
. So,