Chemistry, 11.10.2019 02:20 roneesmith2016
The expression of the theoretical yield (ty) in function of limiting reagent (lr) of a reaction is as follows: ty = ideal mole ratio of (target product / lr) x #mol(lr) x mw(target product) the ideal mole ratio is the one provided by the equation of the reaction. if a reaction uses (4.50x10^0) g of aniline and 1.25 times as many ml of acetic anhydride as the number of grams of aniline, what is the theoreticl yiled of acetanilide (mw = 135.17 g/mol) in the reaction?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:00
Use examples from the article to explain one positive and one negative effect that chemistry has had on society
Answers: 2

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
The expression of the theoretical yield (ty) in function of limiting reagent (lr) of a reaction is a...
Questions




Chemistry, 19.05.2021 02:50




Mathematics, 19.05.2021 02:50

Arts, 19.05.2021 02:50


Mathematics, 19.05.2021 02:50

Computers and Technology, 19.05.2021 02:50

Mathematics, 19.05.2021 02:50

Mathematics, 19.05.2021 02:50






Physics, 19.05.2021 03:00

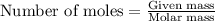 .....(1)
.....(1)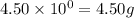 (We know that:
(We know that: 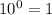 )
)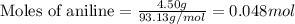
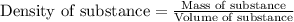
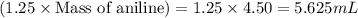
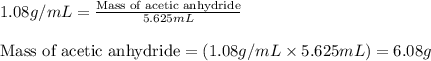
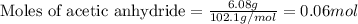
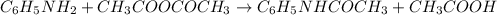
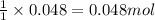 of acetic anhydride
of acetic anhydride