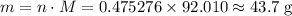50.0 g of n2o4(g) is placed in a 2.0 l evacuated flask and the system is allowed to reach equilibrium according to the reaction: n2o4(g) ⇄ 2 no2(g) kc = 0.133 after the system reaches equilibrium, 5.00 g of no2(g) is injected into the vessel, and the system is allowed to equilibrate once again. calculate the mass of n2o4 in the final equilibrium mixture.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1
You know the right answer?
50.0 g of n2o4(g) is placed in a 2.0 l evacuated flask and the system is allowed to reach equilibriu...
Questions












Biology, 18.10.2019 23:00

Geography, 18.10.2019 23:00

Health, 18.10.2019 23:00






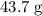 .
.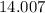 ;O:
;O: 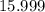 .
.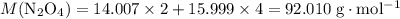 .
.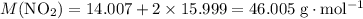 .
.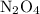 in that sample of
in that sample of 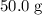 :
: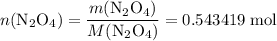 .
. in that
in that 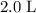 container:
container:  .
.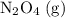 be
be 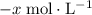 .
.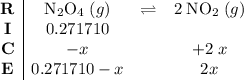 .
.![\displaystyle \frac{[\rm NO_2]^{2}}{[\rm N_2O_4]} = \rm K_{c} = 0.133](/tpl/images/0308/9684/7a7f2.png) .
.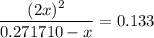 .
. .
. 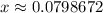 .
.![\rm [N_2O_4] \approx 0.271710 - 0.0798672 = \rm 0.191843 \;mol\cdot L^{-1}](/tpl/images/0308/9684/a5112.png) .
.![\rm [NO_2] \approx 2 \times 0.0798672 = 0.159734\; mol\cdot L^{-1}](/tpl/images/0308/9684/f286e.png) .
.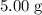 of
of ![\rm[NO_2]](/tpl/images/0308/9684/e223a.png) if it was added to an evacuated
if it was added to an evacuated 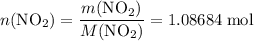 .
.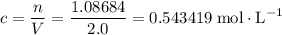 .
.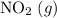 will become
will become 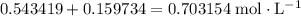 .
.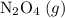 be
be 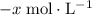 .
.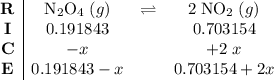 .
. 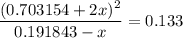 .
.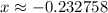 .
.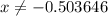 for if that would lead to a negative value for the concentration of
for if that would lead to a negative value for the concentration of 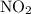 .
.![[{\rm N_2O_4}] = 0.703154 + 2(-0.232758) = \rm 0.237638\; mol\cdot L^{-1}](/tpl/images/0308/9684/8370c.png) .
.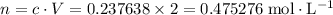 .
.