
Chemistry, 11.10.2019 03:00 Rainey1664
Naoh was titrated to the phenolphthalein endpoint with a known concentration of hcl. the student conducting the experiment placed 12.97 ml of 2.74 m hcl in a beaker with the indicator. before beginning the reaction, he noted that the buret read a volume of 4.50 ml. after reaching the endpoint, the buret read 13.65 ml. calculate the molarity of naoh that reacted upon reaching the endpoint. be sure to report your answer to the appropriate number of significant figures.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
You know the right answer?
Naoh was titrated to the phenolphthalein endpoint with a known concentration of hcl. the student con...
Questions

History, 21.11.2019 09:31


Geography, 21.11.2019 09:31


Biology, 21.11.2019 09:31


Mathematics, 21.11.2019 09:31

Mathematics, 21.11.2019 09:31





Mathematics, 21.11.2019 09:31



History, 21.11.2019 09:31

History, 21.11.2019 09:31



Mathematics, 21.11.2019 09:31

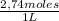 = 0,0347 moles HCl ≡ 0,0347 moles NaOH
= 0,0347 moles HCl ≡ 0,0347 moles NaOH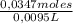 = 3,65M
= 3,65M

