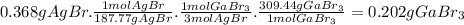Chemistry, 11.10.2019 02:30 iamabouttofail
Asolid mixture weighs 0.6813 g. it contains gallium bromide (gabr3) and other inert impurities. when the solid mixture was dissolved in water and treated with excess silver nitrate (agno3), 0.368 g of agbr was precipitate. a balanced chemical equation describing the reaction is provided below. gabr3(aq) + 3 agno3(aq) ⟶ 3 agbr(s) + ga(no3)3(aq) what is the percent of mass of gabr3 in the solid mixture?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
You know the right answer?
Asolid mixture weighs 0.6813 g. it contains gallium bromide (gabr3) and other inert impurities. when...
Questions

Mathematics, 11.12.2021 17:40

History, 11.12.2021 17:50

Chemistry, 11.12.2021 17:50




World Languages, 11.12.2021 17:50

Mathematics, 11.12.2021 17:50


History, 11.12.2021 17:50

Business, 11.12.2021 17:50


Mathematics, 11.12.2021 17:50


Biology, 11.12.2021 17:50

English, 11.12.2021 17:50


Social Studies, 11.12.2021 17:50