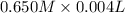Chemistry, 11.10.2019 02:30 katiebotts18
Assume that you have 1, 2, 5, and 10 ml pipets and a 25 ml volumetric flask only. describe in detail how you would prepare a solution with a concentration near 0.100 m from a 0.650 m stock solution using the glassware listed above. calculate the final concentration of the diluted solution (it won't be exactly 0.100 m). it should be within 10% of 0.100 m. ( see the lab skills for the accuracy on volumetric pipets and flasks.)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2


Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1
You know the right answer?
Assume that you have 1, 2, 5, and 10 ml pipets and a 25 ml volumetric flask only. describe in detail...
Questions

Social Studies, 09.10.2019 08:00


Chemistry, 09.10.2019 08:00

Mathematics, 09.10.2019 08:00




History, 09.10.2019 08:00


Physics, 09.10.2019 08:00


Mathematics, 09.10.2019 08:00





Mathematics, 09.10.2019 08:00


Mathematics, 09.10.2019 08:00

Computers and Technology, 09.10.2019 08:00

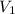 = 25 ml,
= 25 ml, 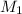 = 0.10 M
= 0.10 M = x ml =
= x ml = 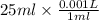 ,
, 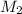 = 0.650 M
= 0.650 M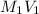 =
= 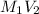
 =
= 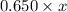
 =
=