
Chemistry, 11.10.2019 03:30 mdndndndj7365
Consider the following reaction and select the false statement below.
10 i−(aq) + 16 h+(aq) + 2 mno4−(aq) → 5 i2(s) + 2 mn2+(aq) + 8 h2o(l)
(a) this is a reduction-oxidation (redox) reaction
(b) the iodine ion is the reducing agent
(c) the permanganate ion is the oxidizing agent
(d) the hydrogen ion is neither reduced nor oxidized
(e) in the reaction as written, ten moles of electrons are transferred from the oxidizing agent to the reducing agent

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
You know the right answer?
Consider the following reaction and select the false statement below.
10 i−(aq) + 16 h+(aq) +...
10 i−(aq) + 16 h+(aq) +...
Questions



Health, 08.07.2021 01:50

Mathematics, 08.07.2021 01:50


Mathematics, 08.07.2021 01:50






Chemistry, 08.07.2021 01:50

Mathematics, 08.07.2021 01:50

Mathematics, 08.07.2021 01:50

Health, 08.07.2021 01:50


Biology, 08.07.2021 01:50


Arts, 08.07.2021 01:50

Mathematics, 08.07.2021 01:50

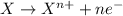
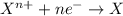
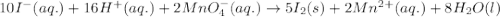
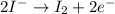 ( × 5)
( × 5)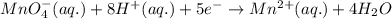 ( × 2)
( × 2)

