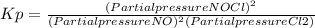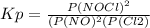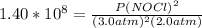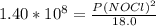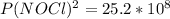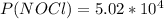Chemistry, 11.10.2019 17:30 BatmanVS1944
2 no(g) + cl2(g) ⇄ 2 nocl(g) kp = 1.40 × 108 a reaction vessel initially contains 3.0 atm of no and 2.0 atm of cl2(g). what is the pressure of no(g) when equilibrium is reached?
3.6 × 10-4 atm
3.8 × 10-8 atm
0.5 atm
2.0 atm
1.0 atm
1.1 × 10-7 atm

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2
You know the right answer?
2 no(g) + cl2(g) ⇄ 2 nocl(g) kp = 1.40 × 108 a reaction vessel initially contains 3.0 atm of no...
Questions






Mathematics, 03.09.2020 20:01

Biology, 03.09.2020 20:01

Physics, 03.09.2020 20:01

English, 03.09.2020 20:01


Mathematics, 03.09.2020 20:01

Computers and Technology, 03.09.2020 20:01

Mathematics, 03.09.2020 20:01


Biology, 03.09.2020 20:01




History, 03.09.2020 20:01