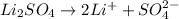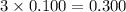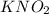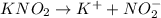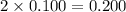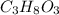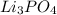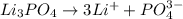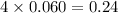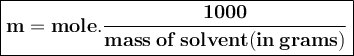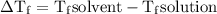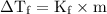Choose the aqueous solution that has the highest boiling point. these are all solutions of nonvolatile solutes and you should assume ideal van't hoff factors where applicable. choose the aqueous solution that has the highest boiling point. these are all solutions of nonvolatile solutes and you should assume ideal van't hoff factors where applicable. a. 0.100 m li₂so₄
b. 0.100 m kno₂
c. 0.200 m c₃h₈o₃
d. 0.060 m li₃po₄e. they all have the same boiling point.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

You know the right answer?
Choose the aqueous solution that has the highest boiling point. these are all solutions of nonvolati...
Questions



English, 28.04.2021 02:30


Computers and Technology, 28.04.2021 02:30



World Languages, 28.04.2021 02:30




Mathematics, 28.04.2021 02:30

Mathematics, 28.04.2021 02:30

Mathematics, 28.04.2021 02:30

Mathematics, 28.04.2021 02:30


Mathematics, 28.04.2021 02:30



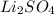
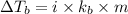
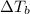 = Elevation in boiling point
= Elevation in boiling point 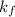 = boiling point constant
= boiling point constant