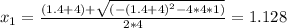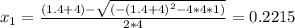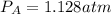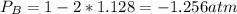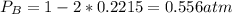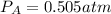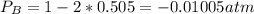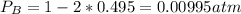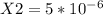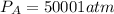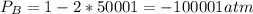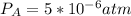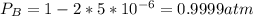Consider the following reaction: a(g)⇌2b(g). find the equilibrium partial pressures of a and b for each of the following different values of kp. assume that the initial partial pressure of b in each case is 1.0 atm and that the initial partial pressure of a is 0.0 atm. make any appropriate simplifying assumptions. kp = 1.4?
kp = 2.0 * 10^-4?
kp = 2.0 * 10^5?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1


Chemistry, 23.06.2019 09:30
What is the force of an object when it landed(sitting in the ground)
Answers: 2
You know the right answer?
Consider the following reaction: a(g)⇌2b(g). find the equilibrium partial pressures of a and b for...
Questions

English, 12.08.2020 20:01


Computers and Technology, 12.08.2020 20:01





Mathematics, 12.08.2020 20:01












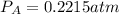
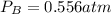
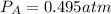
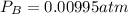
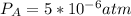
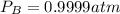
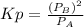
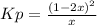
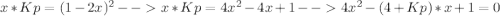
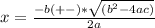 (equation 1)
(equation 1)