
Chemistry, 11.10.2019 23:10 erniewernie
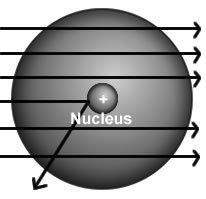
The results of rutherford's gold foil experiment gave him the evidence to arrive at two conclusions: (1) an atom was much more than just empty space and scattered electrons and (2)
a) in any atom, electrons orbit a central nucleus.
b) all atoms were composed of three subatomic particles.
c) an atom must have a positively charged center that contains most of its mass.
d) an atom must have an equal number of positive and negative particles in a central location.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1

Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1
You know the right answer?
The results of rutherford's gold foil experiment gave him the evidence to arrive at two conclusions:...
Questions










Spanish, 18.11.2020 16:50


Physics, 18.11.2020 16:50







Computers and Technology, 18.11.2020 16:50



