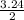Chemistry, 11.10.2019 23:30 redthangracing
2.00 mol of h2(g) and 1.00 mol of i2(g) are placed in a 1.00 l container, and they react to form hi(g). at equilibrium, it is found that 1.80 moles of hi(g) are present in the container. calculate k for the reaction: h2(g) i2(g) ⇄ 2 hi(g)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1


Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
You know the right answer?
2.00 mol of h2(g) and 1.00 mol of i2(g) are placed in a 1.00 l container, and they react to form hi(...
Questions




Biology, 30.06.2019 04:20




Mathematics, 30.06.2019 04:30







English, 30.06.2019 04:30

Computers and Technology, 30.06.2019 04:30

Mathematics, 30.06.2019 04:30

Social Studies, 30.06.2019 04:30


Mathematics, 30.06.2019 04:30

![k = \frac{[[HI]^{2} }{[H2][I2]}](/tpl/images/0311/6358/eab06.png)
![k = \frac{[1.8]^{2} }{[2][1]}](/tpl/images/0311/6358/ea4d9.png)