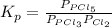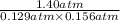Chemistry, 11.10.2019 23:30 sissygirl0807
Phosphorus trichloride gas and chlorine gas react to form phosphorus pentachloride gas: pcl₃(g)+cl₂(g)⇌pcl₅(g). a 7.5-l gas vessel is charged with a mixture of pcl₃(g) and cl₂(g), which is allowed to equilibrate at 450 k. at equilibrium the partial pressures of the three gases are ppcl₃ = 0.129 atm, pcl₂ = 0.156 atm, and ppcl₅ = 1.40 atm. what is the value of kp at this temperature? express your answer using three significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

You know the right answer?
Phosphorus trichloride gas and chlorine gas react to form phosphorus pentachloride gas: pcl₃(g)+cl₂...
Questions

English, 12.01.2021 23:30

French, 12.01.2021 23:30

Mathematics, 12.01.2021 23:30


Mathematics, 12.01.2021 23:30

Chemistry, 12.01.2021 23:30

Mathematics, 12.01.2021 23:30


Mathematics, 12.01.2021 23:30

Mathematics, 12.01.2021 23:30


Advanced Placement (AP), 12.01.2021 23:30


Advanced Placement (AP), 12.01.2021 23:30


Mathematics, 12.01.2021 23:30


Mathematics, 12.01.2021 23:30


Mathematics, 12.01.2021 23:30

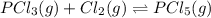
 will be as follows.
will be as follows.