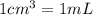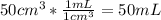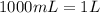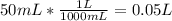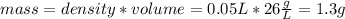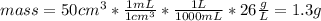Suppose you have 50.0 cm³ of a substance with a density of 26.0 g/l and you want to determine the mass of the substance. the calculation can be broken down into three steps. first, convert the volume from cubic centimeters to milliliters. then, convert the volume from milliliters to liters. finally, determine the mass of the sample in grams. show the unit analysis by placing the correct components into the unit‑factor slots.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3
You know the right answer?
Suppose you have 50.0 cm³ of a substance with a density of 26.0 g/l and you want to determine the ma...
Questions

Mathematics, 11.10.2021 19:50

Chemistry, 11.10.2021 19:50

Biology, 11.10.2021 19:50


Advanced Placement (AP), 11.10.2021 19:50


Chemistry, 11.10.2021 19:50

Biology, 11.10.2021 19:50



Mathematics, 11.10.2021 19:50


History, 11.10.2021 19:50

Mathematics, 11.10.2021 19:50



Geography, 11.10.2021 19:50

World Languages, 11.10.2021 19:50


Biology, 11.10.2021 19:50