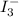The amount of i₃⁻(aq) in a solution can be determined by titration with a solution containing a known concentration of s₂o₂⁻³(aq) (thiosulfate ion). the determination is based on the net ionic equation 2s₂o₃²⁻(aq)+i₃⁻(aq)⟶s₄o₆²⁻(aq)+3i⁻( aq). given that it requires 38.1 ml of 0.440 m na₂s₂o₃(aq) to titrate a 25.0 ml sample of i₃⁻(aq), calculate the molarity of i₃⁻(aq) in the solution.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1


Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1
You know the right answer?
The amount of i₃⁻(aq) in a solution can be determined by titration with a solution containing a know...
Questions

Mathematics, 04.07.2020 17:01

Mathematics, 04.07.2020 17:01




Mathematics, 04.07.2020 17:01

History, 04.07.2020 17:01

Mathematics, 04.07.2020 17:01




Geography, 04.07.2020 17:01

Business, 04.07.2020 17:01







Chemistry, 04.07.2020 17:01

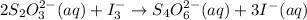
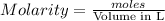
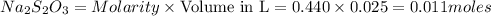
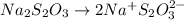
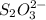 = 0.011
= 0.011 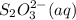 require 1 mole of
require 1 mole of 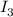
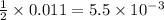 moles of
moles of