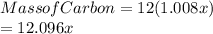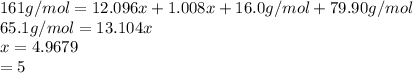Chemistry, 12.10.2019 06:10 whitakers87
Acompound of molar mass 161 g/mol contains only carbon, hydrogen, bromine, and oxygen. analysis reveals that the compound contains 12 times as much carbon as hydrogen by mass. find the molecular formula.

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1


You know the right answer?
Acompound of molar mass 161 g/mol contains only carbon, hydrogen, bromine, and oxygen. analysis reve...
Questions

English, 16.09.2019 01:20

Geography, 16.09.2019 01:20

Mathematics, 16.09.2019 01:20

Mathematics, 16.09.2019 01:20



Biology, 16.09.2019 01:20


Advanced Placement (AP), 16.09.2019 01:20

Biology, 16.09.2019 01:20




Business, 16.09.2019 01:20






Mathematics, 16.09.2019 01:20