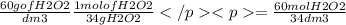Chemistry, 12.10.2019 19:30 kevonmajor
Hydrogen peroxide is sold commercially as an aqueous solution containing approximately 60 g/dm³ of hyrogen peroxide.
calculate the concentration in mol/dm³, of a solution containing 60.0 g/dm³ of hydrogen peroxide.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
Hydrogen peroxide is sold commercially as an aqueous solution containing approximately 60 g/dm³ of h...
Questions

Health, 18.10.2020 05:01

Business, 18.10.2020 05:01




Biology, 18.10.2020 05:01

Chemistry, 18.10.2020 05:01


Mathematics, 18.10.2020 05:01




Social Studies, 18.10.2020 05:01

Geography, 18.10.2020 05:01

Mathematics, 18.10.2020 05:01



Mathematics, 18.10.2020 05:01


Social Studies, 18.10.2020 05:01