Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3


Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
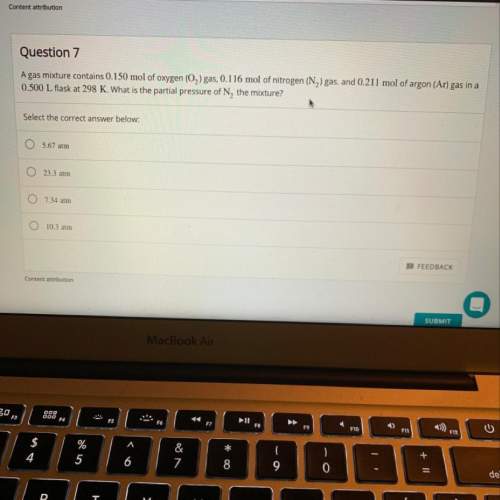
Agas mixture contains 0.150 mol of o2 gas, 0.116 mol of n2 gas, and 0.211 mol of ar gas in a 0.500 l...
Questions




Mathematics, 12.11.2019 09:31


History, 12.11.2019 09:31


Mathematics, 12.11.2019 09:31


Mathematics, 12.11.2019 09:31

Mathematics, 12.11.2019 09:31

Mathematics, 12.11.2019 09:31

Mathematics, 12.11.2019 09:31

Mathematics, 12.11.2019 09:31

Mathematics, 12.11.2019 09:31

Social Studies, 12.11.2019 09:31



Advanced Placement (AP), 12.11.2019 09:31