
Chemistry, 14.10.2019 17:30 alexthebest3976
Which of the following chemical equations does not correspond to a standard molar enthalpy of formation? a. mg(s) + c(s) + 3/2 o_2(g) rightarrow mgco_3(s) b. c(s) + 1/2 o_2(g) rightarrow co(g) c. n_2(g) + o_2(g) rightarrow 2 no(g) d. n_2(g) + 2 o_2(g) rightarrow n_2o_4(g) e. h_2(g)+l/2 o_2(g) rightarrow h_2o(l)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Imagine that you’re getting ready to move to a new city. when people move, they are influenced by push factors and pull factors, and you have many reasons for your move. which of the following factors is an example of a pull factor? a. wanting to move because you’ve found a great new school somewhere new b. needing to move because there are not enough resources in your old hometown c. being forced to move because your old home is gone d. having to move because there are no jobs in your current hometown
Answers: 1

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1


Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3
You know the right answer?
Which of the following chemical equations does not correspond to a standard molar enthalpy of format...
Questions


Spanish, 12.10.2019 16:20


Mathematics, 12.10.2019 16:20


Biology, 12.10.2019 16:20

English, 12.10.2019 16:20


Biology, 12.10.2019 16:20


History, 12.10.2019 16:20

Mathematics, 12.10.2019 16:20

Social Studies, 12.10.2019 16:20


Biology, 12.10.2019 16:20



Social Studies, 12.10.2019 16:20

Biology, 12.10.2019 16:20

Mathematics, 12.10.2019 16:20

 + O₂
+ O₂ → 2NO
→ 2NO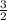 O
O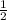 O₂
O₂


