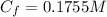Chemistry, 14.10.2019 18:10 leilanimontes714
The conversion of cyclopropane to propene in the gas phase is a first-order reaction with a rate constant of 6.7 x 10-4 s -1 at 500 c. cyclopropane (ch2ch2ch2) -- ch3-ch=ch2 (propene) if the initial concentration of the reactant was 0.25 m, what is the concentration after 8.8 min?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 23.06.2019 02:00
What is the source of continuous heat and energy that we receive from the sun
Answers: 2
You know the right answer?
The conversion of cyclopropane to propene in the gas phase is a first-order reaction with a rate con...
Questions



History, 22.05.2020 09:00

Biology, 22.05.2020 09:00

Biology, 22.05.2020 09:00








Biology, 22.05.2020 09:01



Mathematics, 22.05.2020 09:01




Chemistry, 22.05.2020 09:01