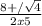Chemistry, 14.10.2019 21:00 missdee6929
H2(g) + i2(g) â 2 hi(g) kc = 64 at 400âºc 3.00 moles of h2 and 3.00 moles of i2 are placed in a 4.00 l container and the system is allowed to reach equilibrium. calculate the concentration of hi at equilibrium.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2
You know the right answer?
H2(g) + i2(g) â 2 hi(g) kc = 64 at 400âºc 3.00 moles of h2 and 3.00 moles of i2 are placed in a 4.00...
Questions

Social Studies, 13.10.2020 14:01

Health, 13.10.2020 14:01


Biology, 13.10.2020 14:01

Mathematics, 13.10.2020 14:01


Mathematics, 13.10.2020 14:01

Mathematics, 13.10.2020 14:01

Mathematics, 13.10.2020 14:01

Computers and Technology, 13.10.2020 14:01

Mathematics, 13.10.2020 14:01

History, 13.10.2020 14:01

Mathematics, 13.10.2020 14:01

Mathematics, 13.10.2020 14:01

English, 13.10.2020 14:01


Social Studies, 13.10.2020 14:01



Chemistry, 13.10.2020 14:01

![Kc = \frac{{D}^dx[C]^c}{[A]^ax[B]^b}](/tpl/images/0319/5603/47fdd.png)
![Kc = \frac{[HI]^2}{[H2]x[I2]}](/tpl/images/0319/5603/fe065.png)