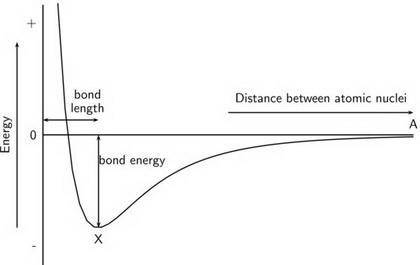
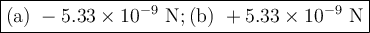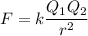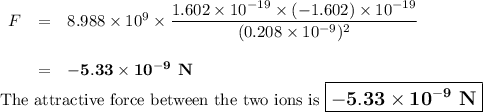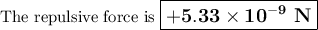Chemistry, 14.10.2019 22:00 tylrmannon
The atomic radii of li+ and 02 ions are 0.068 and 0.140 nm, respectively. (a) calculate the force of attraction between these two ions at their equilibrium interionic separation (i. e., when the ions just touch one another). (b) what is the force of repulsion at this same separation distance? 2.19 for a k+-c- ion pair, attractive and repulsive energies ea and er, respectively, depend on the distance between the ions r, according to

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1


Chemistry, 23.06.2019 06:30
Aplanet similar to earth has four moons roughly the same distance away. the moon that will most affect tides on the planet is the one that has the greatest a) mass. b) volume. c) density. d) amount of water.
Answers: 1
You know the right answer?
The atomic radii of li+ and 02 ions are 0.068 and 0.140 nm, respectively. (a) calculate the force of...
Questions

Mathematics, 19.06.2020 00:57





English, 19.06.2020 00:57


Mathematics, 19.06.2020 00:57

Computers and Technology, 19.06.2020 00:57


History, 19.06.2020 00:57

Chemistry, 19.06.2020 00:57


English, 19.06.2020 00:57

Mathematics, 19.06.2020 00:57