
Chemistry, 14.10.2019 22:10 foriegnngal
At 900 k the following reaction has kp=0.345: 2so2(g)+o2(g)ââ2so3(g) in an equilibrium mixture the partial pressures of so2 and o2 are 0.125 atm and 0.470 atm , respectively.
what is the equilibrium partial pressure of so3 in the mixture?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2
You know the right answer?
At 900 k the following reaction has kp=0.345: 2so2(g)+o2(g)ââ2so3(g) in an equilibrium mixture the...
Questions


Social Studies, 17.02.2021 18:10


History, 17.02.2021 18:10

English, 17.02.2021 18:10

History, 17.02.2021 18:10


Physics, 17.02.2021 18:10

Social Studies, 17.02.2021 18:10

Mathematics, 17.02.2021 18:10

Mathematics, 17.02.2021 18:10


Health, 17.02.2021 18:10

English, 17.02.2021 18:10



Mathematics, 17.02.2021 18:10

History, 17.02.2021 18:10

Mathematics, 17.02.2021 18:10

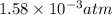
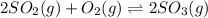 The expression for equilibrium constant for this reaction will be,
The expression for equilibrium constant for this reaction will be,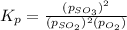
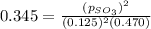
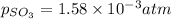
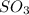 in the mixture is
in the mixture is 


