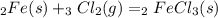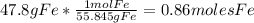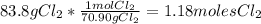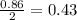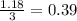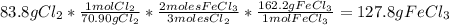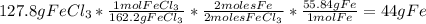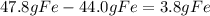Chemistry, 15.10.2019 00:00 nihadsalim10
For the following reaction, 47.8 grams of iron are allowed to react with 83.8 grams of chlorine gas. iron (s) + chlorine (g) iron(iii) chloride (s) what is the maximum amount of iron(iii) chloride that can be formed? grams what is the formula for the limiting reagent? subscriptsuperscript what amount of the excess reagent remains after the reaction is complete? grams

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1

Chemistry, 23.06.2019 00:30
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
For the following reaction, 47.8 grams of iron are allowed to react with 83.8 grams of chlorine gas....
Questions







Social Studies, 31.07.2019 22:00



Social Studies, 31.07.2019 22:00







Mathematics, 31.07.2019 22:00

Mathematics, 31.07.2019 22:00

Mathematics, 31.07.2019 22:00

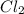
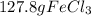 are formed
are formed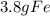 remain after the reaction is complete
remain after the reaction is complete