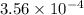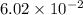Ammonia can be produced via the chemicalreaction
\rm n_2 (g) + 3 h_2 (g) \rightleftharpoons 2 nh_3 (g)
during the production process, the production engineer determinesthe reaction quotient to be q_2 = 3.56ã10â4. ifk_2 = 6.02ã10â2, what can besaid about the reaction?
1)the reaction has reached equilibrium.
2)the reaction is not at equilibrium and willproceed to the left.
3)the reaction is not at equilibrium and willproceed to the right.
4)the reaction is not at equilibrium, but it isnot possible to determine whether the reaction needs to proceedright or left to reach equilibrium.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1


Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
Ammonia can be produced via the chemicalreaction
\rm n_2 (g) + 3 h_2 (g) \rightleftharpoons 2...
\rm n_2 (g) + 3 h_2 (g) \rightleftharpoons 2...
Questions


English, 25.03.2021 04:50

Mathematics, 25.03.2021 04:50

Mathematics, 25.03.2021 04:50

Mathematics, 25.03.2021 04:50

English, 25.03.2021 04:50

Mathematics, 25.03.2021 04:50

Mathematics, 25.03.2021 04:50

Mathematics, 25.03.2021 04:50

Mathematics, 25.03.2021 04:50


Health, 25.03.2021 04:50

Biology, 25.03.2021 04:50




English, 25.03.2021 04:50


Mathematics, 25.03.2021 04:50