Chemistry, 15.10.2019 01:00 BustD0wnAnt
1.24 grams of magnesium phosphate tribasic dissolved in 1 l of lemon juice. what is the ksp of the magnesium phosphate tribasic in lemon juice at room temperature?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1


Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1
You know the right answer?
1.24 grams of magnesium phosphate tribasic dissolved in 1 l of lemon juice. what is the ksp of the m...
Questions

History, 18.10.2019 22:30

Biology, 18.10.2019 22:30










Mathematics, 18.10.2019 22:30



Biology, 18.10.2019 22:30



English, 18.10.2019 22:30

History, 18.10.2019 22:30

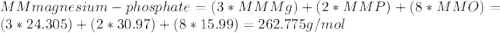
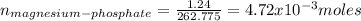
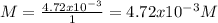
![K_{s} =[Mg^{2+}]^{3}[PO_{4}^{3-} ]^{2} =(3s)^{3} (2s)^{2}](/tpl/images/0320/1996/28dc2.png)